We are a new program aiming to group traumatic and degenerative insults to the nervous system and their clinical implications in single conceptual paradigms. This approach allows us to capture and exploit recent molecular developments in neuronal and axonal death and import them to a broad range of clinical problems in the traumatic-neurodegenerative range.
Our approach is clinicopathological and translational (Fig. 1).
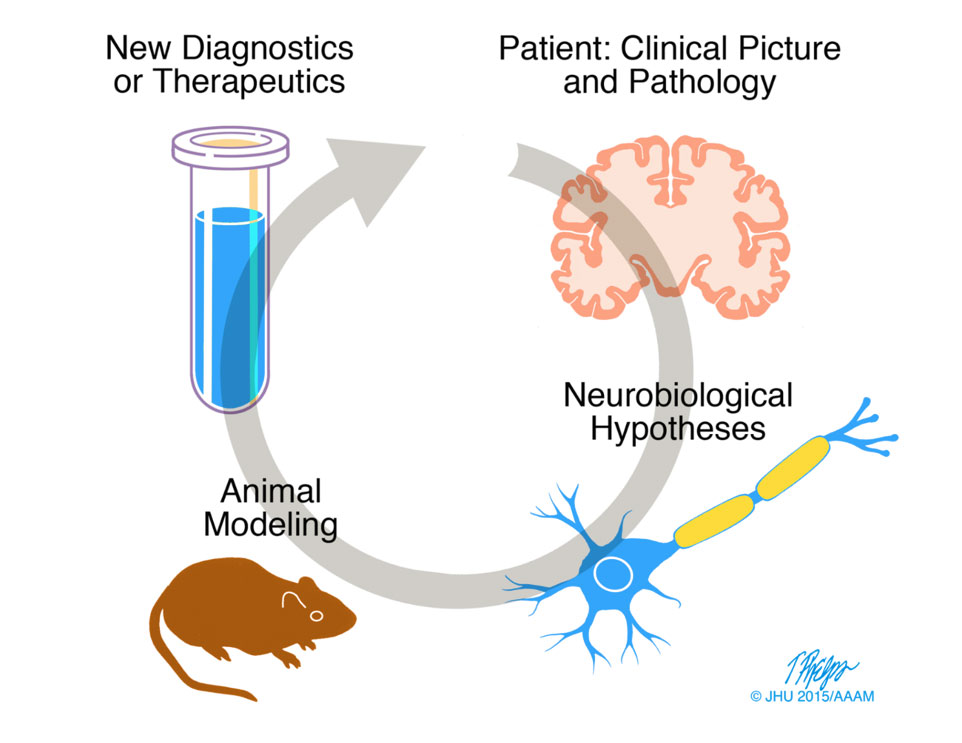
We start from clinical presentations, identify key problems, model them in animals and in vitro systems, explore mechanisms and molecular therapeutic targets, and then revert back to the bedside with novel diagnostic or therapeutic approaches.
Traumatic and non-traumatic brain injury
Traumatic brain injury (TBI) is a serious public health problem. There are about 2.8 million new cases of (TBI)-related ED visits, hospitalizations, and deaths in the US every year, most of them from falls, struck-by or struck-against events, and motor vehicle accidents. Although most cases of TBI are simple concussions with only brief changes in mental status that usually improve over a few weeks, a significant number is associated with permanent brain damage and, in some cases, progressive neurological disease.
Not all forms of brain injury are TBI. Another common type of injury is hypoxia-ischemia, from respiratory suppression or poor oxygenation of the brain or from interruption of blood flow to the brain, focal or diffuse. There is also brain injury from toxins (toxic encephalopathy), metabolic impairments, and low vitamin levels (e.g. B1, B12).
Brain injury: gateway and model of neurodegenerative disease
Of special interest to our program are links between brain injury, especially TBI, and neurodegenerative disease. One reason is secondary degeneration stemming from TBI, for example brain atrophy following severe DAI, CTE developing after repeat concussions, and possibly Alzheimer’s disease (AD). Besides their obvious clinical significance, such links raise important neuropathological questions such as transsynaptic neuronal cell death and the relationship between axonopathy and protein aggregation in the brain (proteinopathy)(Fig. 2).
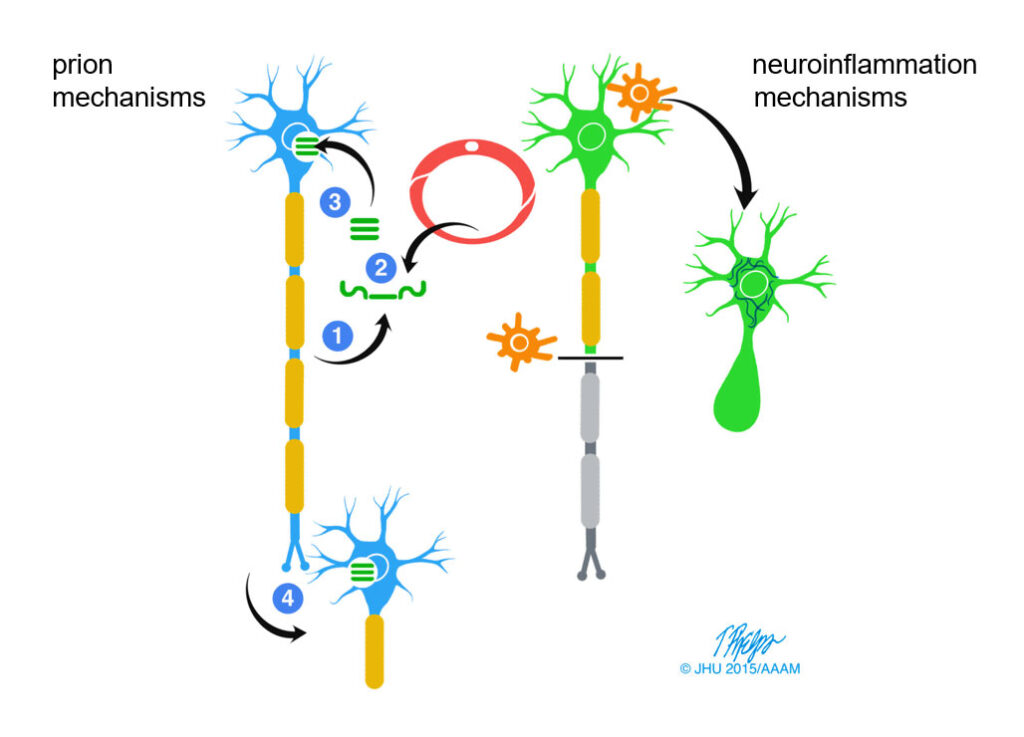
Another reason is that TBI is itself a model of degeneration: because the time of onset is clearly delineated we can follow the course and detail of neural responses, complex adaptive changes known as “neuroplasticity” and failures thereof, and processes related to pathological mechanisms central to neurodegenerative diseases, such as neuroinflammation, proteinopathy, and neuronal cell death.
Investigators at BIDR have separate interests in major neurodegenerative diseases including Dr. Koliatsos (AD and motor neuron disease), Dr. Alexandris (AD and Lewy body dementias), Dr. Wong (AD, motor neuron disease, frontotemporal lobar degeneration), Drs. Troncoso and Morris (all major neurodegenerative diseases of brain and spinal cord), and Dr. Martin (motor neuron disease).
Axons and white matter: the problem of vulnerability and new prospects for protection and treatment
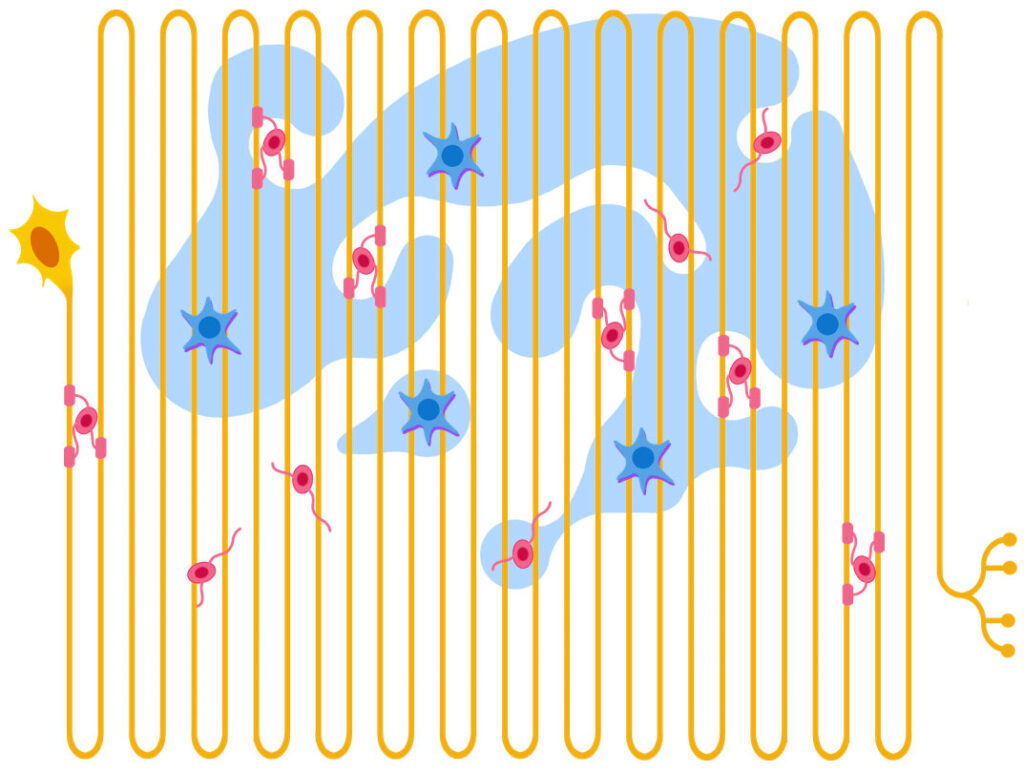
A central feature of our program is our emphasis on axons and the white matter, a thus-far neglected area of query that plays central roles not only in TBI and anoxic-ischemic injury, but also in toxic and infectious encephalopathies, demyelinating disorders, and in the early stages of major neurodegenerative diseases. The importance of white matter (and white-matter dependent associative cortex) is increasing in significance as we ascend from simple to complex mammalian species all the way to humans.
The unique anatomy of axons that contain the bulk of neuronal cytoplasm in long tracts makes them more vulnerable to different types of injury (Fig. 4). At the same time, increasing evidence of the independence of axons from the cell body of neurons and the revolutionary discovery of signals governing axonal maintenance and demise, such as the universal redox cofactor NAD+ and the NADase SARM1, is transforming our view on white matter to a very active and plastic part of the nervous system with unprecedented translational opportunities.